Rare Melanocortin-4 Receptor Pathway Diseases: An Urgent and Growing Concern
Hyperphagia (pathological, insatiable hunger) and early-onset obesity (presence of obesity before age 5, and BMI ≥95th percentile for age and sex) are distressing features of rare melanocortin-4 receptor (MC4R) pathway diseases1,2 which have a profound impact on both patients and caregivers’ lives.3,4
Understanding hyperphagia: Pathological, insatiable hunger – what does it really mean?
Hyperphagia can cause overwhelming disruption to both patients and caregivers from very early in life.3,4,5

Psychological distress

Social isolation

Fractured relationships

Difficulty attending school

Reduced ability to focus during school

Family guilt and stigma
Testimonials
"We have nobody that we can really talk to about it. My side of the family doesn’t even see anything wrong with him… he is just perfect the way he is, and I guess they don’t want to deal with the situation or accept it."
"We didn’t like to go places. And if you go to somebody’s house, trying to keep them away from the chips and dip is so hard, it’s easier to just not go."
"I felt very agitated and very sad a lot of the time…I just really didn’t have many friends… even many friends to hang out with and…I don’t know. I just kind of felt alone in a sense."
"I feel like people are like ‘why don’t they get that girl on a diet’ and I am just scared that she is going to go through all of that and as a parent, I am constantly worried about people judging us that don’t know, you know, that my daughter does have this syndrome."
Caregiver
Caregiver
Patient
Caregiver
Real-life burden and unmet needs for patients and families
Early-onset obesity as a result of hyperphagia in rare MC4R pathway diseases can lead to consequences similar to those seen in childhood obesity.3
Children and adolescents with obesity are:6
more likely to have pre-diabetes
more likely to have asthma
more likely to have high blood pressure
more likely to have fatty liver disease
...than those with a healthy weight.

Early intervention is important to relieving the extensive burden of hyperphagia, and managing the impact of future comorbidities associated with early-onset obesity.5,7
Which is why it is vital to understand the root cause of rare MC4R pathway diseases and be able to identify the clinical features.
An Impaired Hunger Signalling Pathway
The MC4R pathway in the hypothalamus is critical for regulating energy balance, appetite and body weight. Genetic variants in the MC4R pathway can lead to a deficiency in α–melanocyte-stimulating hormone (α-MSH), which can lead to impaired MC4R signalling, a disrupted energy balance and an impossibility to reach satiety.2,8
These genetic disruptions can cause hyperphagia and obesity from early childhood.1,2
The MC4R pathway: function, disruption & impact on hyperphagia
Identify Rare Melanocortin-4 Receptor Pathway Diseases Early and Transform Lives
Recognise Key Clinical Features Early to Change Lives
Early Identification of the clinical features of rare MC4R pathway diseases can help aid early diagnosis and move children living with this condition onto their appropriate care path as early as possible, helping them to achieve an overall improved quality of life.9,10
Alongside the cardinal symptoms of hyperphagia and early-onset obesity, MC4R pathway diseases can have various other clinical manifestations:1,11
Click here to learn more about BBS
Learn moreBardet-Biedl syndrome (BBS):
- Polydactyly
- Early-onset retinal dystrophy
- Kidney anomalies / dysfunction
Monogenic disease:
- Light or pale skin colour for ethnicity
- Red hair
- Hypogonadotropic hypogonadism
Click here to learn more about monogenic disease
Learn more
Got a specific question?
If you can't find what you're looking for, or want to discuss a topic in more detail, reach out to our team.
Earlier Diagnosis Is Possible: Refer for Genetic Testing Today
In the European Reference Network 2024 Bardet-Biedl syndrome consensus statement and recommendations, genetic testing is recommended to inform early diagnosis and appropriate interventions for patients living with rare MC4R pathway diseases who exhibit clinical features such as hyperphagia (pathological, insatiable hunger) and/or early-onset obesity, and/or a family history of obesity.11

If you suspect your patient has a rare MC4R pathway disease they should be immediately referred for genetic testing.
The results of genetic testing can have a profound impact on both a patient’s life, and beyond, by:7
Enabling access to appropriate care.5,12
Reducing social stigma by using a diagnosis to dispel misconceptions of ‘laziness’, ‘lacking willpower’ and ‘self-discipline’.1
Helping the individual and carers to make informed decisions.13
Identifying more pathogenic variants implicated with rare MC4R pathway diseases, expanding knowledge and supporting future patient care.9
Advances in genetic testing mean genetic variants that cause rare MC4R pathway diseases can be diagnosed earlier than ever before.13
genetic variants in the MC4R pathway are associated with hyperphagia and obesity, with evidence showing that variations in genes such as POMC, PCSK1, LEPR, and SH2B1 within the pathway can influence weight.8,14
genes identified as a potential cause of BBS, more than 20 have been associated with MC4R pathway function.15

What the experts have to say: Genetic Testing
Learn about treatment for patients with rare MC4R pathway diseases
Providing the latest research and information
Visit the content hub to access a variety of educational materials, including presentations from international experts, infographics and downloadable handouts.
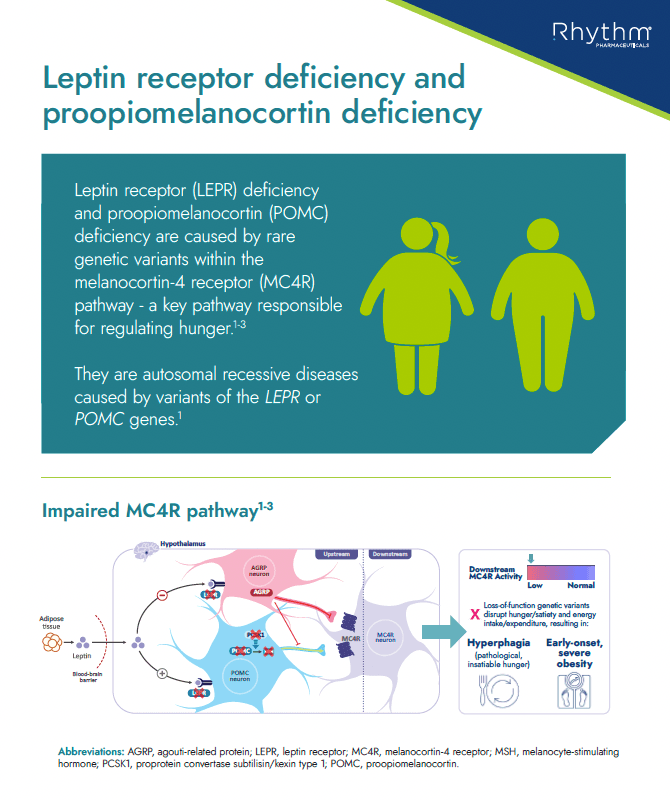
Educational information about LEPR and POMC deficiency, including what it is, key characteristics/clinical features in patients with these conditions, prevalence and route to diagnosis
Handout
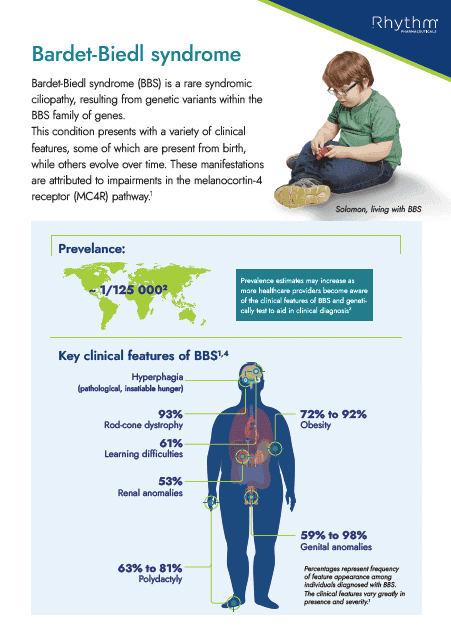
Educational information about Bardet-Biedl syndrome (BBS), its association with obesity, characteristics and route to diagnosis
Handout
References:
- 1. Malhotra S, et al. J Pediatr Genet. 2021;10;194–203
- 2. Clément K, et al. Physiology Behavior. 2020;227:113134.
- 3. Forsythe E, et al. Orphanet J Rare Dis. 2023;18:181
- 4. Heymsfield SB, et al. Obesity (Silver Spring). 2014;22:S1‒S17.
- 5. Forsythe E, et al. Orphanet J Rare Dis. 2023;18(1):182
- 6. Sharma V, et al. Obes Reviews. 2019;20:1341–1349
- 7. Poitou C, et al. Eur J Endocrinol. 2020;183;R149–R166
- 8. Yazdi F, et al, PeerJ, 2015;3:e856.
- 9. Huvenne H, et al. Obes Facts. 2016;9:158–73
- 10. Eneli I, et al. Appl Clin Genet. 2019;12:87‒93.
- 11. Dollfus, H, et al. Eur J Hum Genet. 2024; 32, 1347–1360
- 12. Kühnen P, et al. Orphanet J Rare Dis. 2022;5;17(1):38
- 13. Loos R, Yeo G. Nat Rev Genet. 2022;23;120–133
- 14. Novelli G, et al. Nutrients. 2023; 15(12): 2782
- 15. Forsyth RL, et al. Bardet-Biedl Syndrome Overview. In: Adam MP, et al. editors. GeneReviews® [Internet]. Seattle (WA): University of Washington, Seattle; 1993–2023.